The global medical device industry is currently navigating a paradox. On one hand, devices are becoming “intelligent,” and on the other, the operational risks associated with these devices are hitting historic highs.
In 2024, the U.S. medical device sector recorded 1,059 recall events, an 8.6% increase over the previous year and the highest total in four years. These events didn’t just impact a few niche products; they affected over 440 million units.
But the numbers only tell half the story. The nature of these failures has changed. “Device Failure” and “Software Malfunctions” are among the primary causes of recalls.
In high-velocity environments, the traditional “paper-and-spreadsheet” recall process is dangerously slow. When a safety alert triggers, hospital staff cannot afford to cross-reference lot numbers against PDF lists manually.
This is where the QR Code changes the equation, as a dynamic access layer for device intelligence.
In this article, we will thoroughly explore the significant role of QR Codes within the medical device sector.
Table of contents
- Where QR Codes fit in FDA vs. EU rules
- The two-code problem
- The GS1 digital link revolution
- Why your QR Code solution must be intelligent
- QR Code implementation checklist
- Where Uniqode fits in a regulated QR Code architecture
- Frequently asked questions
Where QR Codes fit in FDA vs. EU rules
If you are manufacturing for a global market in 2026, you are likely wrestling with two diverging regulatory philosophies. A “global pack” label—one single design that works everywhere—is becoming a strategic puzzle.
The United States
The FDA’s Unique Device Identification (UDI) system remains the global gold standard for traceability. Under 21 CFR Part 830, every medical device must carry a UDI in two forms:
- Human Readable Interpretation (HRI): The text alphanumeric string.
- Automatic Identification and Data Capture (AIDC): A machine-readable barcode.
A common misconception is that you can simply “swap” your existing barcode for a standard consumer QR Code.
This is false.
The FDA delegates the technical standards to accredited issuing agencies, such as GS1. For the primary regulatory UDI on the device itself (especially for surgical instruments or implants), GS1 standards often mandate the GS1 DataMatrix.
A standard consumer QR Code lacks the specific data structure required for the primary UDI in these space-constrained, direct-marking applications.
The European Union
While the U.S. remains conservative regarding label formats, the EU is aggressively modernizing content delivery.
As of July 2025, Commission Implementing Regulation (EU) 2025/1234 has radically expanded the eligibility for electronic Instructions for Use (eIFU). Previously reserved for implants, eIFU is now permitted for nearly all devices intended for “Professional Use.”
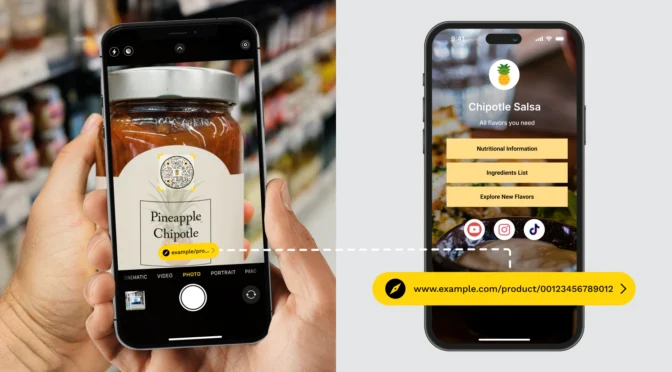
This is a game-changer. It allows manufacturers to remove the bulky, expensive multi-language paper booklets from their packaging, provided they place a digital carrier, specifically a QR Code, on the label that links directly to the instructions.
This creates a “Dual-Track” reality. Your label requires a high-density DataMatrix to meet the FDA’s tracking requirements. Still, it also needs a QR Code to unlock the EU’s paperless benefits and ensure accessibility for users without hospital-grade barcode scanners.
The two-code problem
This regulatory split has quietly introduced a new packaging reality for global manufacturers: label clutter.
To satisfy both traceability mandates and digital information requirements, many manufacturers now place two different 2D codes on the same package—a GS1 DataMatrix for UDI, inventory, and recall traceability, alongside a standard QR Code for electronic Instructions for Use (eIFU), safety updates, or supplemental content.
On paper, this seems manageable. In practice, it introduces real clinical risk.
| Consider a high-pressure trauma unit. A nurse grabs a suture pack and scans it to log usage in the hospital’s Electronic Health Record (EHR) system. The workflow is designed to be fast, automated, and safe. If the nurse scans the GS1 DataMatrix, the system correctly reads the GTIN and lot number. The device is logged, expiration and recall status are verified, and the workflow continues uninterrupted. But in the rush of care, the nurse accidentally scans the neighboring QR Code instead. The scanner reads a simple URL—something like www.brand.com/manual. The EHR system, expecting a structured UDI string, rejects the scan with an error. Faced with an alert tone and no time to troubleshoot, the nurse bypasses scanning altogether and manually enters the serial or lot number. |
This is where patient safety begins to erode.
Manual data entry undermines the UDI system’s safeguards, breaking traceability. The issue isn’t the presence of QR Codes, but their lack of clinical intelligibility, causing ambiguity when similar codes have different functions in critical workflows.
If a UDI DataMatrix and a QR Code coexist, they need clear functional separation. The UDI DataMatrix must be on the regulatory panel. The QR Code must be in a separate, labeled information zone or on another package face. Use the ISO 15223-1 eIFU symbol on the QR Code to indicate its purpose and prevent misuse.
But ultimately, this two-code compromise is a symptom and not a solution.
And that brings us to the next evolution: how GS1 Digital Link and intelligent QR architectures eliminate this trade-off.
The GS1 digital link revolution
A GS1 Digital Link encodes regulated product identifiers, such as the GTIN, lot number, or serial number, directly into a web-based URL. When embedded in a QR Code, it transforms that symbol into something fundamentally new: a single code that behaves like a UDI barcode for machines and a dynamic information gateway for humans.
How does it work?
Instead of the traditional “element string” (e.g., (01)1234…), the data is encoded as a web-compatible Uniform Resource Identifier (URI).
Traditional UDI: (01)09501101530004(10)LOT123(17)251231
GS1 Digital Link: https://id.med-device.com/01/09501101530004/10/LOT123/17/251231
In clinical systems, GS1-aware scanners can extract structured UDI data offline, just as they would from a DataMatrix. At the same time, clinicians, distributors, or regulators can scan the same code with a mobile device to access instructions for use, safety notices, recall updates, or regulatory documentation without adding label clutter or workflow ambiguity.
This architecture is also considered to be future-proof. Under GS1’s Sunrise 2027 initiative, QR Codes powered by Digital Link are set to replace legacy linear barcodes globally.
Enterprise-grade QR Code solutions platforms, like Uniqode, enable manufacturers to implement GS1 Digital Link at an enterprise scale, combining compliance, content governance, uptime assurance, and dynamic control in one system.
For a deeper dive, read → Explainer: GS1 QR Code with Digital Link
Why your QR Code solution must be intelligent
Whether you are using a standard QR Code for eIFU or piloting a GS1 Digital Link, the infrastructure behind the code is as critical as the code itself. In the medical device world, a broken link isn’t just a 404 error, a “Misbranding” violation that can trigger an FDA Warning Letter.
A. Dynamic kill switches for recalls
When a recall hits, speed is the only metric that matters. McKinsey estimates that a single major recall can cost a manufacturer up to $600 million.
With a dynamic QR Code management system, you can update the destination of the QR Code in real-time without reprinting the packaging.
- Normal State: Code links to the eIFU.
- Recall State: If a specific batch (Lot XYZ) is recalled, the manufacturer can update the link for that specific batch to redirect to a “STOP – RECALLED” warning page with return instructions. This capability prevents the device from being used even if it is sitting on a hospital shelf, effectively “quarantining” the product digitally.
B. Version control
EU regulations for implants require eIFUs to remain accessible for 15 years. Furthermore, you must provide access to historical versions of the IFU that correspond to the device’s manufacturing date.
An intelligent QR platform allows you to manage these version histories seamlessly. You can ensure that a device manufactured in 2023 links to the 2023 version of the manual, even if the current 2025 manual is different. This maintains the integrity of the device’s “Digital Twin.”
C. Security & HIPAA compliance
If your QR Code workflow involves patient interaction (e.g., patient implant cards or home-care device instructions), you are touching the realm of Protected Health Information (PHI).
Using a free or generic QR Code generator is a security risk. Medical device manufacturers require platforms that are SOC 2 Type 2 certified and HIPAA compliant, ensuring that any data exchange is encrypted, auditable, and secure against tampering.
Related → Free vs. Paid QR Code Generators
QR Code implementation checklist
Implementing QR Codes in a regulated environment requires more than just generating a link. It requires a validated process that aligns with ISO verification standards and hospital IT infrastructure.
Here is the technical roadmap for manufacturers modernizing their labeling strategy.
Step 1: Audit label real estate & eIFU eligibility
Before redesigning packaging, conduct a regulatory audit of your SKU portfolio to identify “Label Clutter” and cost-saving opportunities.
- Filter by “Professional Use”: Under EU Regulation 2025/1234, devices intended exclusively for professional use are now eligible for eIFU. Move these SKUs to a “Paperless” workflow immediately to recover label space and reduce packaging weight.
- Calculate Data Density: Measure the available whitespace on your smallest unit-of-sale. If your current DataMatrix and human-readable text occupy >40% of the label face, you are at risk of “Quiet Zone” violations. Switching to a single GS1 Digital Link QR Code can consolidate regulatory data and the eIFU link into one square, freeing up critical real estate.
Step 2: Enforce Error Correction Level H (High)
Medical environments are hostile to printed labels. In the Operating Room, packaging is frequently subjected to abrasion, saline splashes, Betadine stains, and blood spatter.
- Avoid Level L and M: Standard retail QR Codes use Level L (Low) or M (Medium) error correction, which recovers only 7-15% of damaged data. If a surgeon smears blood across a Level L code, it becomes unreadable.
- Mandate Level H: Configure your printing software to Error Correction Level H. This devotes approximately 30% of the code’s data capacity to Reed-Solomon error correction algorithms.
- The Trade-off: Level H increases the data density (making the “dots” smaller or the code larger). To maintain ISO Grade B scannability, ensure your minimum module width (X-dimension) remains above 0.015 inches (0.38mm) for reliable scanning on standard handhelds.
Step 3: Validate “Resolver” infrastructure & uptime
In a GS1 Digital Link architecture, the QR Code points to a web URL. If that server goes down, your product is effectively “misbranded” under FDA regulations because the safety information is inaccessible.
- Require 99.99% Uptime: Your redirect service (Resolver) must operate with Tier 1 high-availability standards. A “404 Not Found” error during a surgical procedure is a reportable safety event.
- 15-Year Archival Policy: EU regulations for implants require eIFUs to remain accessible for 15 years. Ensure your QR management platform supports “Version History” routing. A scan of a device manufactured in 2023 must resolve to the 2023 version of the IFU, not the 2026 version, to ensure the clinician sees instructions relevant to that specific unit.
Step 4: Validate EHR parsing
The ultimate test of your QR Code is not a smartphone; it is a hospital’s barcode scanner tethered to an Electronic Health Record (EHR) system.
- Test for “Clean Data”: Modern EHRs like Epic (via the “Bridges” interface) and Cerner (CareAware) use parsing logic to extract the GTIN and Expiry Date automatically.
- The Failure Mode: If you use a generic URL generator (e.g., bit.ly/device-manual), the EHR scanner will reject the data because it lacks the standard GS1 Application Identifiers.
- The Fix: Ensure your QR string follows the GS1 URI syntax strictly (e.g., …/01/GTIN/10/LOT…). Conduct physical test scans with hospital partners to verify that the scanner successfully parses the string into the correct fields (Inventory, Patient Record, Billing) without manual data entry.
Where Uniqode fits in a regulated QR Code architecture
Once a QR Code is printed on regulated packaging, it becomes part of the device’s compliance surface, subject to uptime, auditability, version control, and security expectations that extend for years.
This is where an enterprise-grade platform like Uniqode fits; not as a QR Code generator, but as the control layer behind regulated QR deployments.
Uniqode enables manufacturers to manage dynamic QR Code behavior centrally, updating destinations, enforcing version alignment, and responding to recalls, without altering printed labels.
Beyond control, Uniqode also offers detailed analytics. Scan-level visibility, by geography, device type, time, or lot, enables manufacturers to validate that information is accessible in the field, identify gaps in adoption, and detect abnormal scan patterns that may indicate misuse or operational issues.
Smart rules add a further layer of intelligence. Based on predefined logic, the same QR Code can resolve differently depending on context, ensuring clinicians, regulators, and patients each receive information appropriate to their role without introducing ambiguity at the point of care.
Furthermore, Uniqode is designed for regulated environments, with SOC 2 Type II–certified infrastructure, encrypted data handling, and audit-ready controls, capabilities required when QR Codes intersect with clinical workflows or patient-facing use cases.
Frequently asked questions
1. What is the difference between a GS1 Digital Link and a standard QR Code?
A GS1 Digital Link is a standards-based URL that embeds GS1 identifiers (GTIN, lot, expiry) into the link itself. When encoded in a QR Code, it works as both a machine-readable UDI for supply-chain systems and a dynamic link to regulated digital content.
A standard QR Code contains only a basic URL. It carries no GS1 identifiers, is not scannable by clinical or inventory systems for UDI, and is limited to simple consumer-facing use cases.
2. Can I replace the GS1 DataMatrix on my medical device with a QR Code?
No. For FDA and EU UDI compliance, the GS1 DataMatrix is mandatory for primary device identification, especially for direct marking and small labels. QR Codes, including GS1 Digital Link QR Codes, can be used only as a supplement, not as a replacement, where UDI is required.
3. Are QR Codes on medical devices HIPAA compliant?
QR Codes themselves are neutral carriers and are not inherently HIPAA compliant or non-compliant. Compliance depends entirely on the infrastructure behind the code.
Enterprise platforms like Uniqode support HIPAA-ready workflows with HTTPS encryption, audit logs, access controls, and SOC 2 Type II–certified infrastructure.
4. How do dynamic QR Codes help with medical device recalls?
Dynamic QR Codes enable real-time recall responses without requiring the reprinting of packaging. Manufacturers can instantly redirect affected lots to recall warnings, quarantine instructions, or return workflows, ensuring clinicians and patients always access the most current safety information. This dramatically improves recall speed, traceability, and compliance across the entire device lifecycle.